What Is An Apheresis Port?
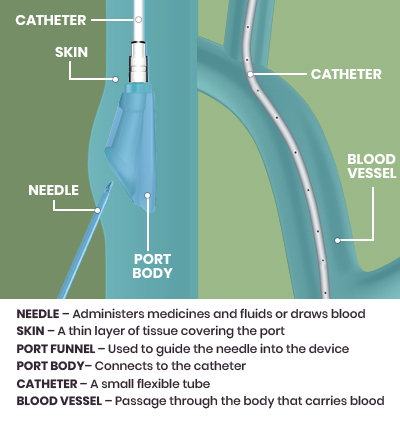
The PowerFlow™ Implantable Apheresis IV Port Port is the first and only* high-flow, power-injectable port designed and indicated specifically for therapeutic apheresis and infusion therapy. The port connects to a small flexible tube, called a catheter, and is inserted into a vein. A special needle with a catheter (called an IV catheter) is inserted into the funnel of the port so that blood can be withdrawn and medicines can be delivered.
*as of Oct. 2024
Where is a PowerFlow™ IV Port placed?
The port is placed beneath your skin during a short, minor surgical procedure. The small tube, called a catheter, is placed inside a vessel that takes blood to your heart. Ports are often placed in the upper chest. Your healthcare provider will place your port where it is best suited for your treatment.
Why might you consider a PowerFlow™ IV Port?
The PowerFlow™ IV Port is specially designed for apheresis procedures. If you require long-term apheresis treatment, the PowerFlow™ IV Port can help provide a reliable access point. Your healthcare provider may also require delivery of medicine or fluids into your bloodstream. If this is the case, the PowerFlow™ IV Port may be an appropriate option for your treatment.
Comfort
Your healthcare team can perform apheresis therapy through your port. They will not need to stick the veins in your arms with a needle. This makes it more comfortable for you.
Long-term health
Catheters placed in larger, central veins allow for faster apheresis treatments and may protect your arm veins from long-term damage caused by repeated needle sticks.
Lifestyle
Implanted ports, compared to other centrally placed vascular access devices, are more likely to permit you to go about your normal day-to-day activities, like showering and swimming. Ask your doctor or nurse about specific activities and the appropriate time to resume them.
Increased privacy and appearance
With an implanted port, there is no exposed device. Implanted ports are small and can be hidden from view. No one needs to know about your treatment unless you want them to.
1 Nagel SN, Teichgräber UK, Kausche S, Lehmann A. Satisfaction and quality of life: a survey-based assessment in patients with a totally implantable venous port system. Eur J Cancer Care (Engl). 2012;21(2):197-204. doi:10.1111/j.1365-2354.2011.01275.x